When ammonium containing sample is merged with a basic (pH > 11,) reagent NH3 is formed. The NH3 passes across a gas-permeable membrane into a acceptor stream containing bromothymol blue in acid (yellow) form. Collection of NH3 in acceptor solution raises pH, and the color change (to blue) monitored by optical fibers at 620nm, is proportional to the initial ammonium content in the sample. By sequencing 100 mcrL of sample, and 100 mcrL 0.1 N NaOH into holding coil, followed by flow reversal of 200 mcrL that positions the reaction mixture into the sensor for reaction rate stopped flow measurement.
Ammonia by Gas Sensor – LOV
2.3.29.
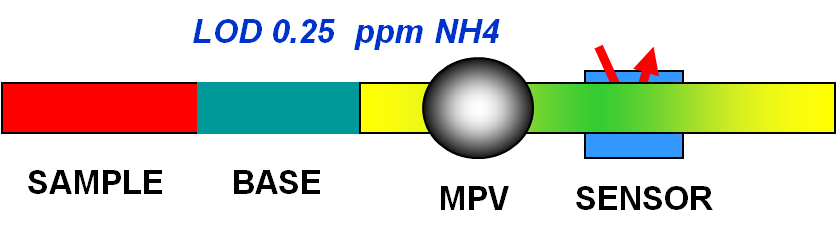
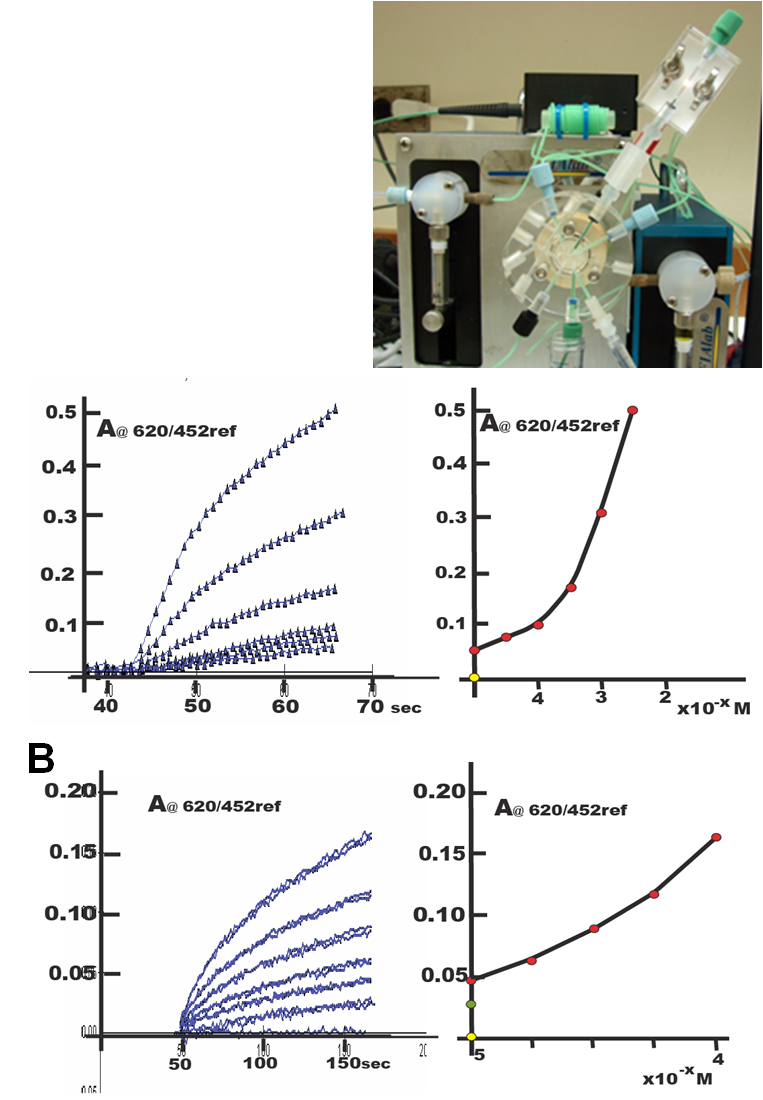
With a stop flow period of 30 seconds the calibration covered range of 0.1 ppm N, which is identical with reagent blank, to 0.5 ppm, 1.0 ppm, 5.0 ppm, 10.0 ppm, 25.0 ppm and 50.0 ppm N. (A). With stop flow period of 120 seconds (B) The sensitivity increased covering the range 0.1, 0.25 , 0.50 ,0.75 and 1.0 ppm N. The yellow point corresponds to instrument background signal, the green point represents the reagent blank. The non linearity of the calibration curve is due to the inherent response of the BTB indicator to small changes within that pH range.









